Cart (0 Items)
Your cart is currently empty.
View Products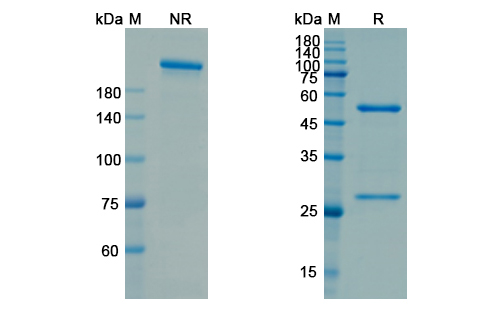
| Size | |
|---|---|
| Isotype | IgG2Kappa |
| Brand | |
| Product type | |
| Clonality | |
| Expression system | |
| Applications |
| Product name | Utomilumab Biosimilar - Anti-Human TNFRSF9 mAb - Research Grade |
|---|---|
| Source | DrugBank DB15113 |
| Species | Human |
| Expression system | Mammalian cells |
| Molecular weight | LC: 24kDa HC: 51kDa |
| Purity | >85% |
| Buffer | PBS pH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | 4°C for short term; -20°C for long term |
| Brand | ProteoGenix |
| Aliases /Synonyms | Utomilumab,Utomilumab,Human TNFRSF9,anti-Human TNFRSF9 |
| Reference | PX-TA1012 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG2Kappa |
| Clonality | Monoclonal Antibody |
Utomilumab is a monoclonal antibody that binds to 4-IBB protein (also known as CD137 protein). 4-IBB protein is a receptor found in different immune cells such as helper T-cells, killer T-cells and natural killer cells. 4-IBB/CD137 protein is a costimulatory receptor whose activation results in a series of events that leads to cytokine secretion and effector functions. Hence, targeting CD137 protein has been linked to antitumor immunity response and overall tumor reduction. Indeed, once utomilumab is injected in the bloodstream its specific binding to CD137 protein stimulates the immune cells which, as a result, enhances the immune response against tumors.
Utomilumab is under investigation as a potential treatment for advanced forms of cancer either alone or in combination with other therapies. Indeed, pre-clinical studies have suggested that the combination of utomilumab with a checkpoint inhibitor such as anti-PD1/anti-PDL1, or other immunotherapies may lead to amplified immune response. This demonstrates utomilumab anti-tumor activity.
Phase 1 study evaluated the outcome of administering utomilumab in combination with rituximab in patients with certain type of non-Hodgkin’s lymphoma (NHL) also known as CD20-positive NHL. Phase I clinical trial results further confirmed the anti-tumor activity of utomilumab. The utomilumab administration with rituximab was well tolerated and none of the patients interrupted the treatment because of treatment-related side effects. No dose-related toxicities were recorded. The safety and anti-tumor activity of this antibody-based therapy was assessed in phase 1b clinical trial. Utomilumab was tested in combination with pembrolizumab for different advanced solid tumors including renal cell carcinoma, head and neck cancer, thyroid cancer, pancreatic cancer, non-small cell lung cancer, small cell lung cancer, colon cancer, sarcoma, thymoma and melanoma. Result showed that the administration of utomilumab together with pembrolizumab to patients with solid tumors was safe. Once again, no toxicity was observed with increased doses of utomilumab. This product is for research use only.

Utomilumab Biosimilar - Anti-Human TNFRSF9 mAb, on SDS-PAGE under reducing and non-reducing condition. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.

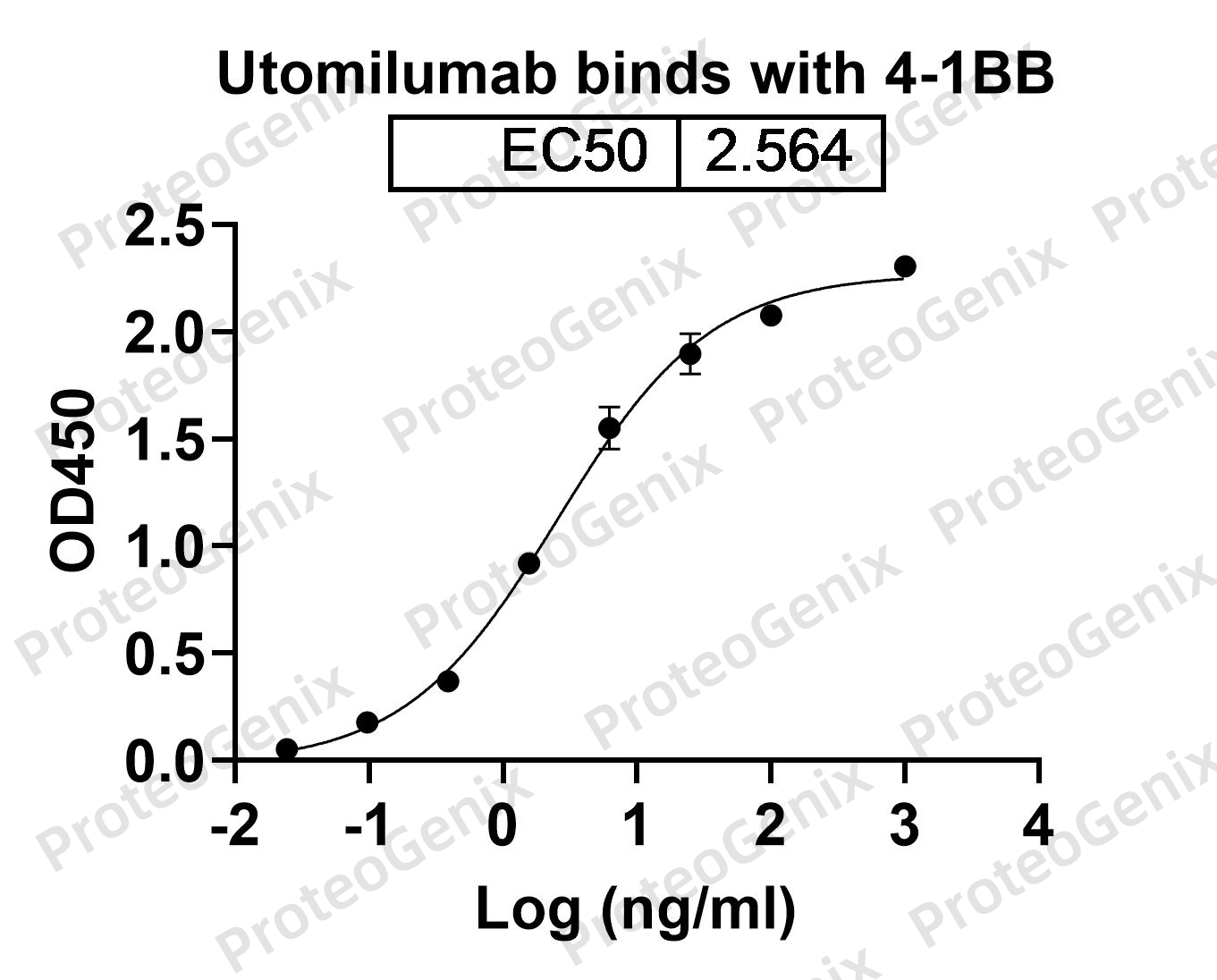
Immobilized CD137 Recombinant Protein (cat. No.PX-P4107) at 0.5µg/mL (100µL/well) can bind to Utomilumab Biosimilar - Anti-Human TNFRSF9 mAb (cat. No.PX-TA1012) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Your cart is currently empty.
View Products


Reviews
There are no reviews yet.