 Antibody production
Antibody production
Are murine monoclonal antibodies still relevant for modern medicine?
Murine monoclonal antibodies were the first antibodies ever to be produced at lab-scale thanks to the discovery of the hybridoma technology in 1975. To date, very few of these antibodies are still in use for therapy due to their low compatibility with the human immune system. However, hybridoma production for therapeutic applications is still widely relevant and frequently carried out in mouse hybridomas. This process generates murine antibodies that serve as frameworks for further antibody humanization processes.
The discovery of murine monoclonal antibodies
Murine monoclonal antibodies are so called due to their origin from rodent hosts, more commonly mice and rats from the Muridae family.
Historically, murine antibodies from the immunoglobulin G class (IgG) have played a crucial role in the development of modern antibody production techniques and they have helped us grasp the potential of the use of these immunoglobulins for therapeutic and analytical applications.
Today, we know that patients treated with native murine antibodies develop an allergic reaction to these molecules termed human anti-mouse or anti-murine antibody (HAMA) response. This response is known to cause a quick elimination of these molecules from the human organism and, therefore, severely limits their therapeutic application.
What causes the HAMA response?
The therapeutic effectiveness of therapeutic antibodies stems from their ability to bind specific antigens, but also from their ability to recruit effector cells and elicit an in vivo biological response.
This double function of the immunoglobin structure results from their globular frameworks which contain two variable regions (Fab), responsible for interacting with the antigen, and a single constant region (Fc), responsible for interacting with the immune cells and the complement cascade.
However, in order to become functional proteins, antibodies need to undergo a complex post-translational modification process that consists in covering the protein framework with N-glycans. This process, termed glycosylation, significantly alters the structure of the antibody and it is essential for antibody recognition by the immune system.
Rodents and humans show important differences in the glycosylation pattern. For instance, mice mostly express the sialic acid N-glycolylneuraminic acid, while humans exclusively express N-acetylneuraminic acid. But interestingly, beyond genetic factors, the pattern of Fc-glycosylation was also shown to depend on environmental factors, such as the frequency and pattern of exposure to immunological challenges.
These differences increase the risks of murine monoclonal antibodies being recognized as foreign molecules by our immune system and often results in quick elimination and weak interaction with our immune cells.
Why is it still relevant to generate murine monoclonal antibodies?
Murine monoclonal antibodies are still recurrently used for a multitude of applications due to the established reputation of the hybridoma technology.
This mature and fully optimized technology allows a cost-effective discovery of high-affinity monoclonal antibodies using mouse germlines.
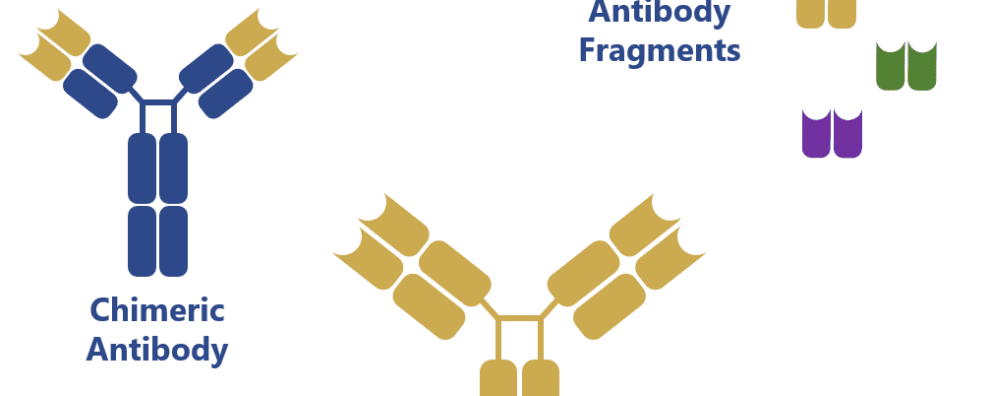
Interestingly, the native form of these antibodies is still in high demand for analytical applications, but, for therapeutic applications, these antibodies serve mostly as the framework for antibody development and engineering techniques consisting in antibody chimerization, humanization and bispecific antibody development from antibody fragments (single-chain antibody fragments, scFvs).
Due to the maturity of the hybridoma technology, it continues to be the leading method for monoclonal antibody discovery. To this date, several of the antibodies currently approved for clinics have originated from mouse hybridoma cell lines which have been humanized or fragmented to increase compatibility towards the human immune system.
Murine monoclonal antibodies engineering
Murine antibodies are often engineered to reduce their natural immunogenicity towards human patients. Initial efforts were prompted by the development of molecular biology techniques that have allowed the sequencing and cloning of antibody sequences and subsequent transfection of other mammalian expression systems.
This flexibility allowed researchers greater control over the antibody sequences and, subsequently, over the immunogenicity burden imposed by native murine monoclonal antibodies.
Genetic manipulation of antibodies first generated chimeric antibody structures containing the mouse Fab regions and a human Fc region. This combination of the regions with different origin allowed to increase the compatibility of these mouse-generated antibodies towards human immune cells.
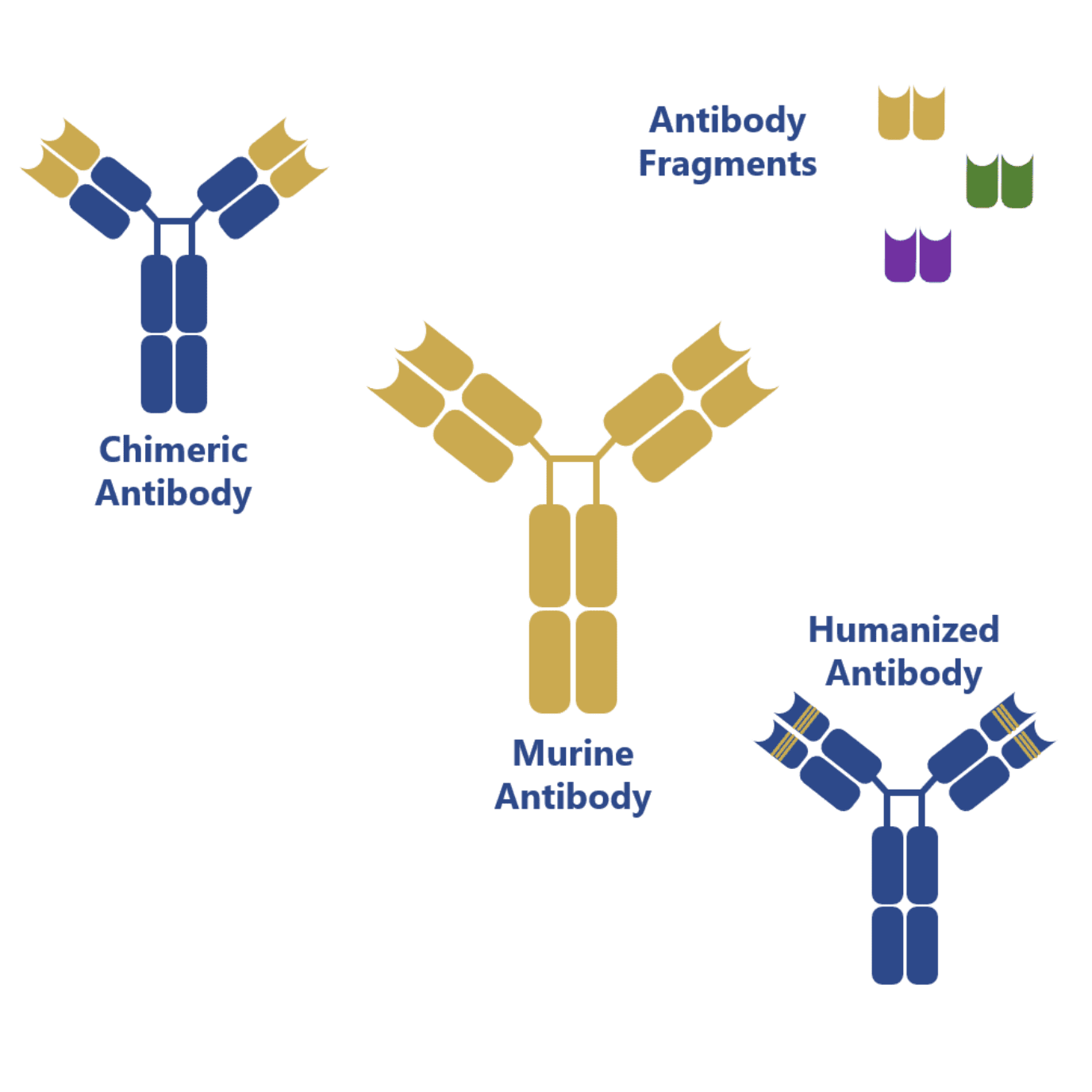
However, more impressive results have been obtained with humanization techniques, which have allowed the targeted mutation of the mouse Fab region in order to increase its homology towards native human antibodies.
Recent advances have also allowed the production of antibody fragments in simpler and less expensive expression systems: bacteria (e.g. Escherichia coli) and yeast (e.g. Saccharomyces cerevisiae). These antibody fragments can be combined in many different ways to form bispecific antibodies (BiTE, diabody, DART, among others) and, because of the lack of the Fc region, they pose a reduced risk of challenging the patient’s immune system and eliciting an immune response.
Concluding remarks
Murine monoclonal antibodies continue to be relevant and recurrently used as frameworks for antibody development techniques. Moreover, due to the maturity and optimized protocols for antibody generation from hybridoma mouse cell lines, this technology and subsequent generation of murine antibodies continue to be a highly popular and cost-effective method to produce new antibodies.
- Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Retrieved from: https://www.antibodysociety.org/resources/approved-antibodies/ Accessed on: October 2019
- Azuma, K. et al. Twin studies on the effect of genetic factors on serum agalactosyl immunoglobulin G levels. Biomed Rep. 2014; 2(2):213-216. doi: 10.3892/br.2014.216
- de Haan, N. et al. The N-Glycosylation of Mouse Immunoglobulin G (IgG)-Fragment Crystallizable Differs Between IgG Subclasses and Strains. Front Immunol. 2017; 8:608. doi: 10.3389/fimmu.2017.00608
- Kaplon, H., Reichert, J. M. Antibodies to watch in 2019. MAbs. 2019; 11(2):219-238. doi: 10.1080/19420862.2018.1556465
- Maureen, S. et al. The Role of Glycosylation in Therapeutic Antibodies. 2011. In: Al-Rubeai M. (eds) Antibody Expression and Production. Cell Engineering, vol 7. Springer, Dordrecht
- Nelson, A. L. et al. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 2010; 9(10):767-774. doi: 10.1038/nrd3229