 Antibody production
Antibody production
How activation mechanisms influence antibody production by B cells in the human organism?
Understanding the mechanisms that influence antibody production by B cells can be invaluable to design more effective immunotherapies. In humans, the mechanisms that elicit the strongest immune responses are those that depend on the linked recognition and cell-cell interaction between two lymphoid-derived cell types: B cells and T cells. In this article, we present the seminal knowledge regarding the origin and development of these cells, as well as their role in the immune response, and how researchers can use that knowledge to design more effective approaches to hybridoma production for therapeutic applications, one of the most relevant technologies for drug discovery.
Key players of the mammalian immune system and their influence in antibody production by B cells
The mammalian immune system comprises an intricate network of interactions between cells that mature from the myeloid or lymphoid cell lineages present in the bone marrow.
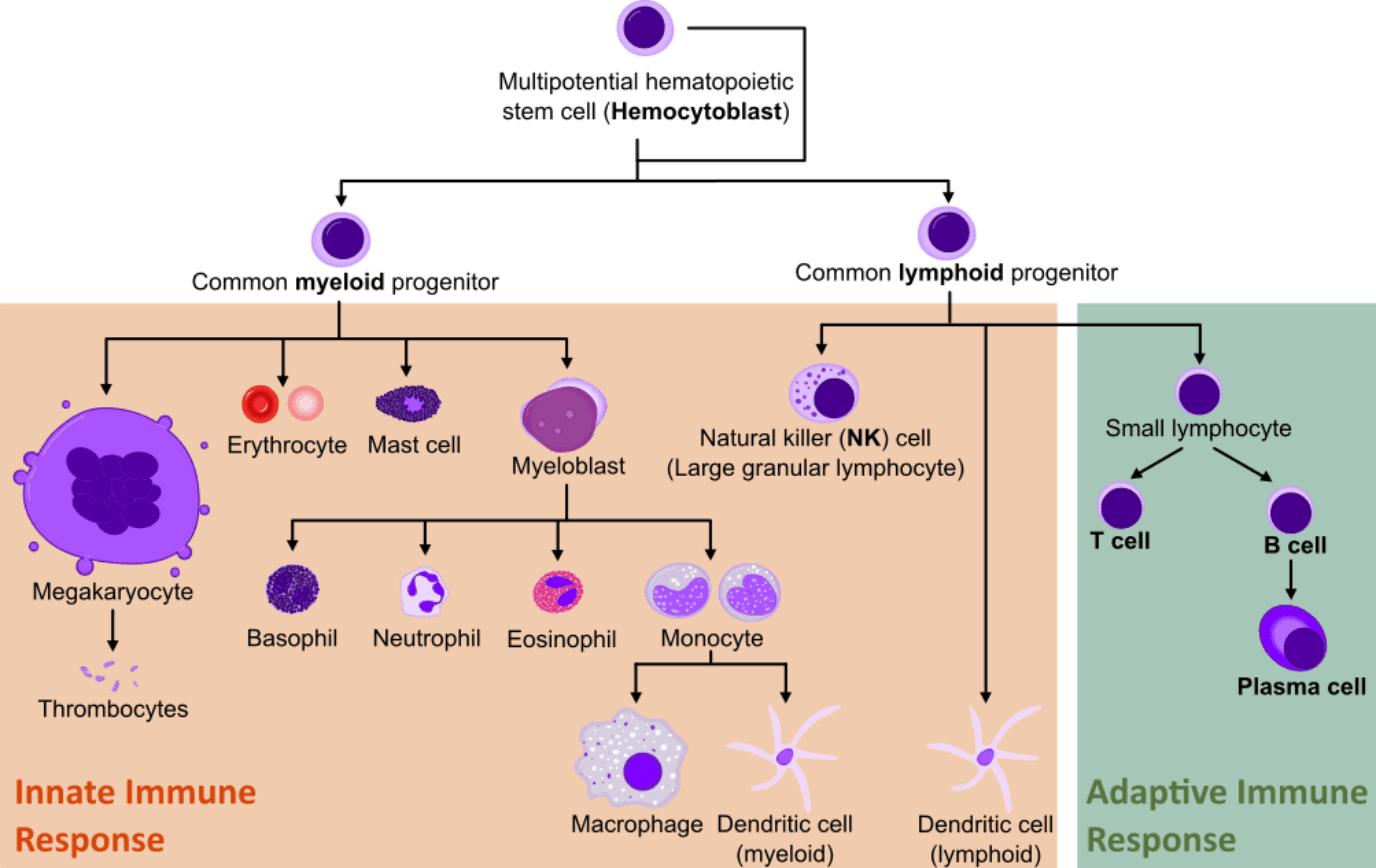
From the multitude of different cell lineages, only lymphocytes can adapt and recognize specific and new foreign molecules in our organism. This comprises the adaptive immune response and it is the basis of many therapeutic immunotechnology strategies.
The first contact with a new antigen catalyzes the beginning of the primary immune response. During this primary contact, there are two key players of the adaptive response: B and T cells. Interestingly, thanks to the production of memory lymphocytes, subsequent exposures to the same antigen will result in a quicker and more effective response, also called the secondary immune response.
Only B cells will eventually differentiate into antibody-secreting effector B cells (plasma cells). But the role of T cells is invaluable for the strong activation and regulation of antibody production by B cells.
T-cell-dependent or independent activation of antibody production by B cells
B cells start their maturation in the bone marrow and complete it in the lymphatic system, while T cells migrate to the thymus for maturation. During the initial stages of B cell maturation, these undergo a process of negative selection where self-reacting clones are eliminated through apoptosis, editing or modification of the receptors. This process minimizes the risk of autoimmunity.
B cells are then activated when they encounter and bind soluble or membrane-bound antigens in their surface receptors (BCR).
In rare cases, B cell activation can be independent of T cell activation (TI). This usually happens when the new antigen is composed of large polysaccharides or lipopolysaccharides, typically found in bacterial capsules. These molecules consist of multiple copies of identical antigenic determinants (polymers) that crosslink the BCR.
They generate a quick immune response but generally result in a weak reaction and often don’t create an immunologic memory.
Interestingly, the most effective immune response is also the most complex. It results from the cell-to-cell interaction between T and B cells challenged by the same antigen. This response is the predominant mechanism mediating the humoral immune response and it is called T-cell-dependent (TD) activation. Unlike independent mechanisms, this type of activation can take up to several days, but the antibodies generated in response to this mechanism have a considerably higher affinity and are more specific than antibodies produced in response to TI activation.
The complexity of TD activation stems from the requirement of co-stimulatory signals. The same antigen needs to interact with both cell types through specific mechanisms. This mechanism is called linked recognition.
It implies that before antibody production by B cells can occur, T cell activation needs to take place. But although the epitope recognized by the helper T cell must be linked to the epitope recognized by the B cell, the two cells don’t need to recognize the same epitope of an antigen. In fact, for more complex natural antigens (e.g. viruses) these two cell types don’t even need to recognize the same protein. Linked recognition requires only that the antigens carried by the two lymphoid cells contain overlapping regions.
This mechanism helps to ensure self-tolerance, by drastically reducing the probability of autoantibody production.
Interestingly, TD activation is not initiated in the focus of the infection, instead, it occurs in the peripheral lymphoid tissues. Invading microorganisms are captured by antigen-presenting phagocytes (the most important of these are dendritic cells), which partially digest and process the pathogens. These cells then transport the antigens to the lymphoid tissues, where they interact with T cells and cause their activation by presenting the processed antigen on their surfaces.
When the activated T cells recognize and bind the antigen on the surface of a B cell, the former is induced to express the CD40 ligand (CD40L) which, in turn, binds to the CD40 protein on the surface of the B cell. This complex, together with other proteins, forms a tight junction between the two cells and results in the polarization of the cytoskeleton of the T cell towards the point of cell-cell contact. Cytokines, specifically interleukin-4, 2 and 5 (IL-4, IL-2, IL-5), are then released at the point of contact, leading to the further activation of the B cell.
This activation is also accompanied by a differentiation process. At this stage, a B cell will either differentiate into a memory cell, storing the immunogenic memory and facilitating the secondary response, or it will differentiate into antibody-secreting effector B cells, also called plasma cells.
Factors that can be used to stimulate antibody production by B cells
The knowledge that our immune system requires linked recognition to induce a strong immune response, has greatly influenced the design of countless immunotherapies, namely vaccine design. For instance, in adults, the immunization against Haemophilus influenzae type B is easily achieved by TI response to the polysaccharide antigens present in the pathogen’s capsule. However, in infants, the TI response is weak due to the immaturity of their immune system and thus insufficient to induce a strong protective response.
In this case, effective vaccines for use in infants are designed to contain the capsular polysaccharide of H. influenzae linked to the tetanus toxoid, a foreign protein able to elicit a very strong TD immune response.
This conjugated vaccine ensures that the polysaccharide that activates the infant’s B cells, is also able to activate its T cells and subsequently generate a strong protective response.
Other factors that have been used to induce TD immune response in vitro are cytokines. For instance, researchers have shown that B cells can be stimulated to proliferate in vitro when exposed to synthetic CD40L and IL-4, the two key components of TD activation. These cells could be maintained for less than 40 days following the treatment with CD40L/IL-4, however, the mechanisms responsible for proliferation arrest after this period have yet to be described. Despite their limited direct application, in vitro studies will continue to be invaluable in helping us understand how to leverage and enhance the immune response of our organism.
Concluding remarks
In our organism, antibody production by B cells is preceded by a complex mechanism of interaction with foreign antigens. Although some polymeric antigens can cause a satisfactory immune response, protein and other complex natural antigens, depend on the interaction with T cells.
This complex mechanism depends on the linked recognition of the antigens that activate B and T cells. In this case, the immune response is strong and leads to the generation of immunogenic memory. Interestingly, a deep knowledge of this mechanism is also guiding researchers in the design of more effective vaccines. Namely, the conjugation of polymeric and protein antigens has shown to be a viable alternative to the use of only polymeric antigens to elicit an adequate primary response in infants.
- Doherty, D. G. et al. Activation and Regulation of B Cell Responses by Invariant Natural Killer T Cells. Front Immunol. 2018; 9:1360. doi: 10.3389/fimmu.2018.01360
- Egbuniwe, I. U. et al. Revisiting the role of B cells in skin immune surveillance. Trends Immunol. 2015; 36(2):102-111. doi: 10.1016/j.it.2014.12.006
- Janeway, C.A. et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10757/
- Lai, A. Y. et al. T and B lymphocyte differentiation from hematopoietic stem cell. Semin Immunol. 2008; 20(4): 207–212. doi: 10.1016/j.smim.2008.05.002
- O’Nions, J. and Allday, M. J. Proliferation and differentiation in isogenic populations of peripheral B cells activated by Epstein-Barr virus or T cell-derived mitogens. J Gen Virol. 2004; 85(Pt 4):881-895. doi; 10.1099/vir.0.19704-0




