 Antibody production
Antibody production
How the phage display technology is boosting the production of valuable bispecific antibodies
Bispecific antibodies are valuable therapeutics due to their unique ability to engage different epitopes or antigens and thus modulate the immune response. But their production remains challenging due to the frequent misassembly of the different antibody chains. Antibody phage display platforms for therapeutic applications may soon change that, as more and more scientists develop unique ways to engineer these antibodies for optimal production.
What are the unique benefits of the phage display technology?
The phage display technology, first developed by George Smith in the 1980s for peptide libraries, and later adapted to the fast screening of antibodies, has earned the Nobel Prize in Chemistry in 2018. Since then, at least 10 antibodies derived from the phage display technology were granted approval by the FDA and EMA for clinical use.
Adalimumab (Humira®) was the first of these biotherapeutics to reach the clinic and, coincidently, also the first fully human antibody ever to be produced in vitro, establishing the phage display technology as one of the main drivers of innovation in clinical research. Adalimumab was designed to targets the tumor necrosis factor alpha (TNF) and has been approved for the treatment of multiple disorders including rheumatoid arthritis, psoriatic arthritis, Crohn disease, among others.
The unparalleled benefits of using the phage display technology for antibody discovery include:
- Fast lead times (only a few weeks are needed to obtain antigen-specific binders by panning antibody libraries via phage display)
- Adapted to the use of toxic or non-immunogenic antigens
- Possibility to tailor an antibody’s cross-reactivity properties according to each project’s needs
- Minimization of animal use in antibody generation
Beyond these unique advantages, phage display technologies continue to push the boundaries of antibody engineering, especially in improving the production of bispecific antibodies. These antibodies, especially promising in the clinical context, are hard to produce in vitro, which has hindered their further development.
Bispecific antibodies are remarkable for their ability to bind two different epitopes on the same or different antigens. In this way, they can engage the immune system and direct the immune response to specific markers. This has proven to be particularly useful in the treatment of cancer where bispecifics engage T cells and direct them towards specific cell surface markers overexpressed on the surface of cancerous cells, which would normally evade the patient’s immune system.
How are bispecific antibodies developed?
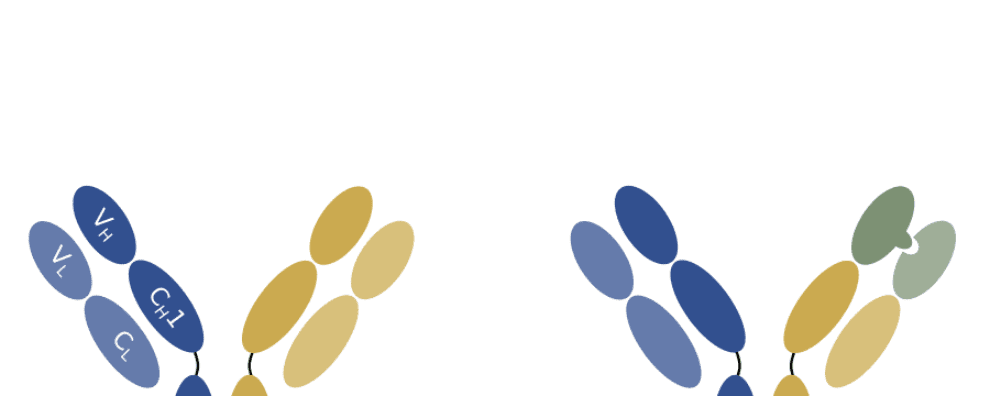
Bispecific antibodies are built by merging two antibodies with affinities to different epitopes/antigens into a single bispecific molecule. But producing these different antibody-encoding genes in a single cell line often leads to chain misassembly, co-expression of undesired antibody pairs, and, subsequently, reduced purity.
One strategy that has been successfully used to overcome this constraint and help bispecific antibodies maintain a native and stable structure is the “knobs-into-holes” amino acid changes. To ensure a correct chain assembly between the two variants, the “knobs-into-holes” strategy bases itself on the rational design of the CH3 (constant heavy chain 3) region to create a “knob” into one of the variants and a hole in the other. This configuration ensures that the bispecific configuration is favored during antibody production and thus increases the purity and yield of the desired bispecific antibody.
However, this strategy does not solve another important challenge in bispecific production – heavy and light chain mispairing between the two variants of the bispecific molecule.
Several strategies have been developed to minimize this issue such as constant domain fusions, chemical ligation, reshaped Fab interfaces, and the use of common light or heavy chains. But these strategies introduce many changes into conserved antibody regions or impose a heavy restriction in chain diversity. These changes are prone to cause an increase in immunogenicity and to limit epitope coverage or antibody affinity.
Future perspectives for bispecific antibody development via phage display technologies
A better method for bispecific antibody development via the phage display technology was recently devised by Daniel Christ and his team at the Garvan Institute of Medical Research (Sydney, Australia). As stated by the authors of the study, despite the great advancement in antibody affinity maturation strategies, the improvement of combinatorial VH-VL chain assembly strategies have remained elusive.
In their recent study, they make use of the phage display technology as well as negative and positive selection strategies to boost the final purity and yield of an IgG bispecific antibody. The elegant solution consists of boosting the affinity between a VH domain and its natural VL pair via random mutagenesis and phage display selection in the presence of a competitor molecule.
Using the humanized Trastuzumab and Pembrolizumab as starting points, the authors engineered a bispecific antibody with a greatly improved production yield (85% final purity, instead of a typical ~50% purity) in the following way:
- Starting from the VH domain from Trastuzumab, the authors developed a VH mutant library by random mutagenesis with an error-prone polymerase
- Panning in suspension was performed in the presence of the biotin-conjugated VL natural pair and of the competitor VL molecule (a germline molecule similar to the natural VL pair but with different CDRs)
- Enrichment of the VH-VL pair with an improved affinity towards each other and sequencing of the most promising pairs
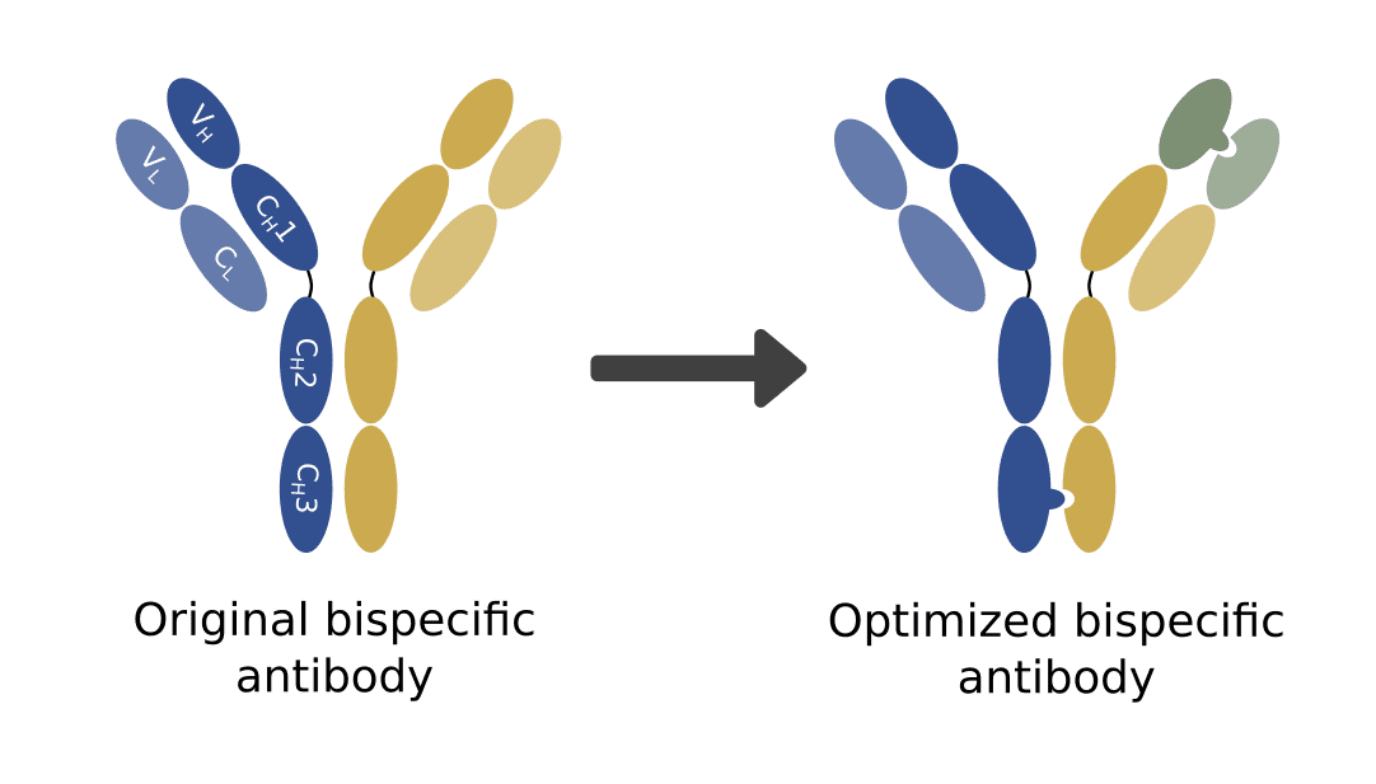
The authors then proceeded to prove that the mutated VH with its natural VL pair retained its affinity towards Trastuzumab’s original antigen. The combined use of the new selection strategy for improved VH-VL specificity and the “knobs-into-holes” strategy for improved assembly of the bispecific configuration resulted in a much lower misassembly ratio and greatly improved production.
This study represents an important step in improving VH-VL pairing and the production of bispecific antibodies. It also opens the door for the further development of rational design strategies that allow us to obtain even better production rates.
Concluding remarks
The correct assembly of VH-VL pairs has remained an important challenge in bispecific antibody production. Misassembly between the two antibody variants as well as errors in VL-VH pair assembly recurrently led to low purity of the desired bispecific configuration and greatly increased purification costs.
Recently, scientists have used the phage display technology to greatly improve the yield and purity of these bivalent molecules. The elegant solution is based on the construction of VH mutant libraries, a biotin-conjugated VL (natural chain pair), and a competitor germline VL to drive the enrichment of VH-VL pairs with improved mutual affinity. This strategy successfully translated into higher purity ratios and minimizes purification costs.
This study opens the door for further improvements in bispecific antibody engineering through the phage display technology.
- Barderas, R. and Benito-Peña, E. The 2018 Nobel Prize in Chemistry: phage display of peptides and antibodies. Anal Bioanal Chem. 2019; 411(12):2475-2479. doi: 10.1007/s00216-019-01714-4
- Carter, P. Bispecific human IgG by design. J Immunol Methods. 2001; 248(1-2):7-15. doi: 10.1016/s0022-1759(00)00339-2
- Joshi, K. K. et al. Elucidating heavy/light chain pairing preferences to facilitate the assembly of bispecific IgG in single cells. MAbs. 2019; 11(7):1254-1265. doi: 10.1080/19420862.2019.1640549
- Luthra, A. et al. Human Antibody Bispecifics through Phage Display Selection. 2019; 58(13): 1701-1704. doi: 10.1021/acs.biochem.9b00037
- Merchant, A. M. et al. An efficient route to human bispecific IgG. Nat Biotechnol. 1998; 16(7): 677-681. doi: 10.1038/nbt0798-677
- Ridgway, J. B. et al. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996; 9(7): 617-21. doi: 10.1093/protein/9.7.617