Cart (0 Items)
Your cart is currently empty.
View ProductsIt looks like you are visiting from outside the EU. Switch to the US version to see local pricing in USD and local shipping.
Switch to US ($)




| Brand | ProteoGenix |
|---|---|
| Product type | Elisa assay kits |
| Isotype | Any isotype |
| Size | 1 Kit, 96 tests |
| Product name | SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Kit |
|---|---|
| Delivery condition | Blue ice (+4°) |
| Storage condition | Stable for 6 months from the date of manufacture if Capture Plate, Controls and HRP conjugated ACE2 are kept at -20°C. The remaining reagents can be stored at 4°C. |
| Brand | ProteoGenix |
| Size | 1 Kit, 96 tests |
| Reference | KPTX02 |
| Note | For research use only. |
| Label or Dye | Horseradish Peroxidase (HRP) |
| Isotype | Any isotype |
| Sample type | Purified Antibody, serum |
| Expected control Results | Positive Control OD450 nm < 0.3 Negative Control OD450nm > 0.9 |
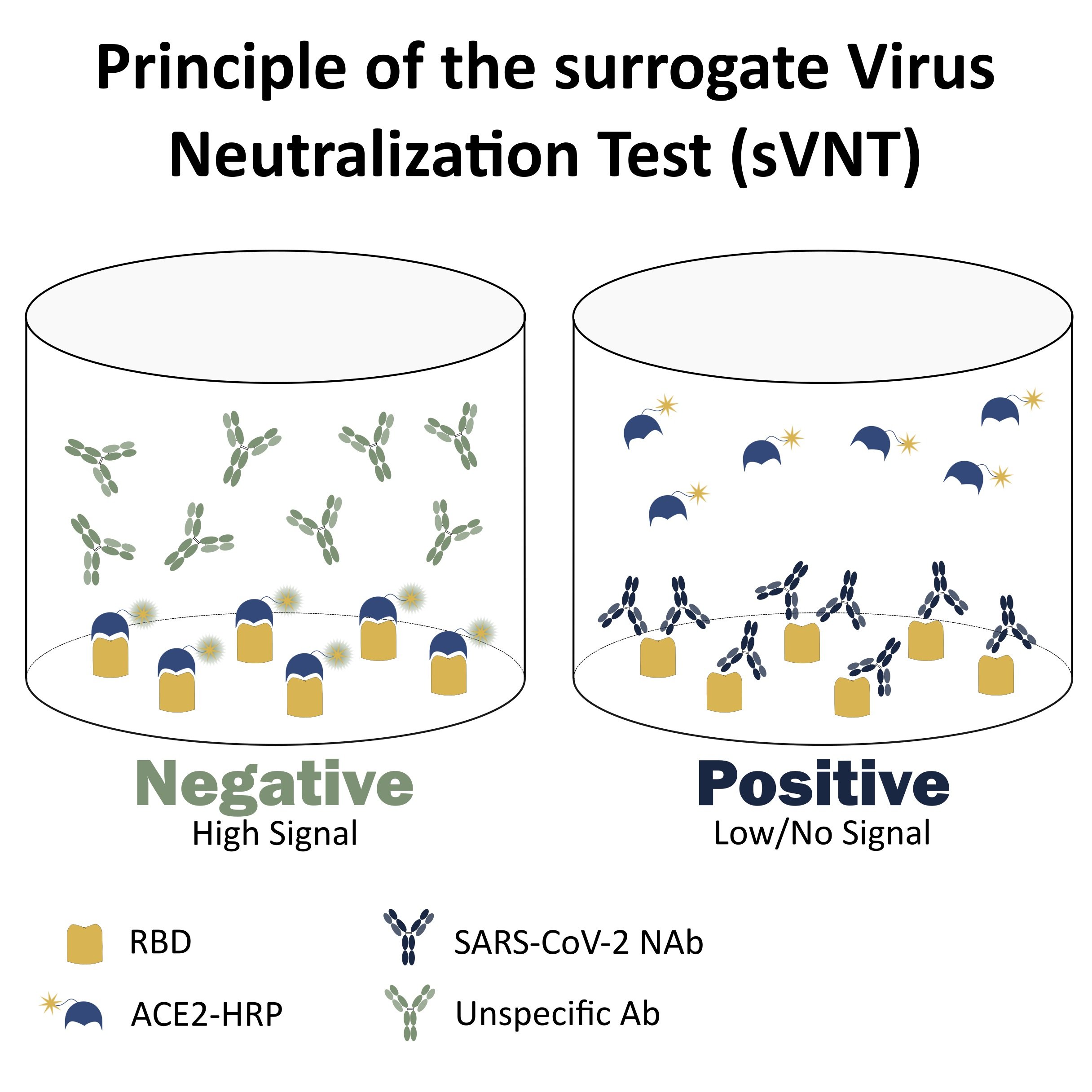
The surrogate Virus Neutralization Test (sVNT) was designed in the versatile ELISA format allowing the quick and parallel screening of multiple purified antibodies and serum samples with potent neutralizing activity. With no species, isotype, or antibody format restriction, it is now possible to perform a preliminary assessment of an antibody or a sample neutralizing potential in less than 2 hours and without needing to use live mammalian cells or native viral particles.
The test works according to the principle of competitive binding between a purified antibody and HRP-labeled human ACE2 (angiotensin-converting enzyme 2), which is the natural ligand of the receptor-binding domain (RBD) in the COVID-19 pathology. For accurate detection, the test kit comprises the following reagents:
• Horseradish peroxidase (HRP) labeled ACE2
• Capture plate coated with unlabeled RBD
• The HRP substrate, TMB (3,3′,5,5′-Tetramethylbenzidine)
• Positive and negative controls
The test works according to the principle of competitive inhibition. In the presence of SARS-CoV-2 neutralizing antibodies (NAbs), the interaction between the RBD and the ACE2 is hampered leading to low or no detectable signal. On the contrary, in the presence of unspecific antibodies (Abs) the formation of the HRT-ACE2-RBD complex takes place leading to an intense signal.
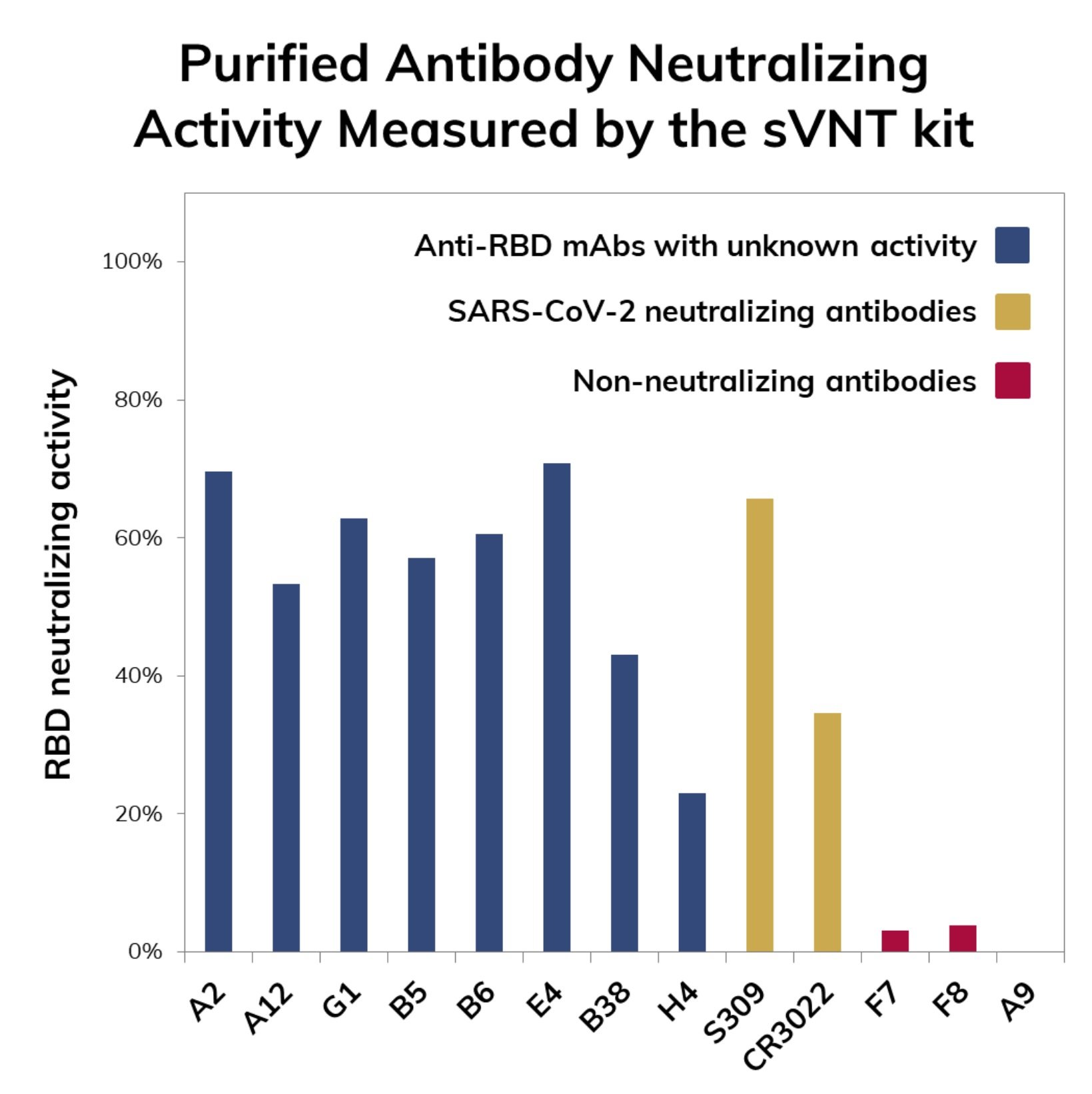
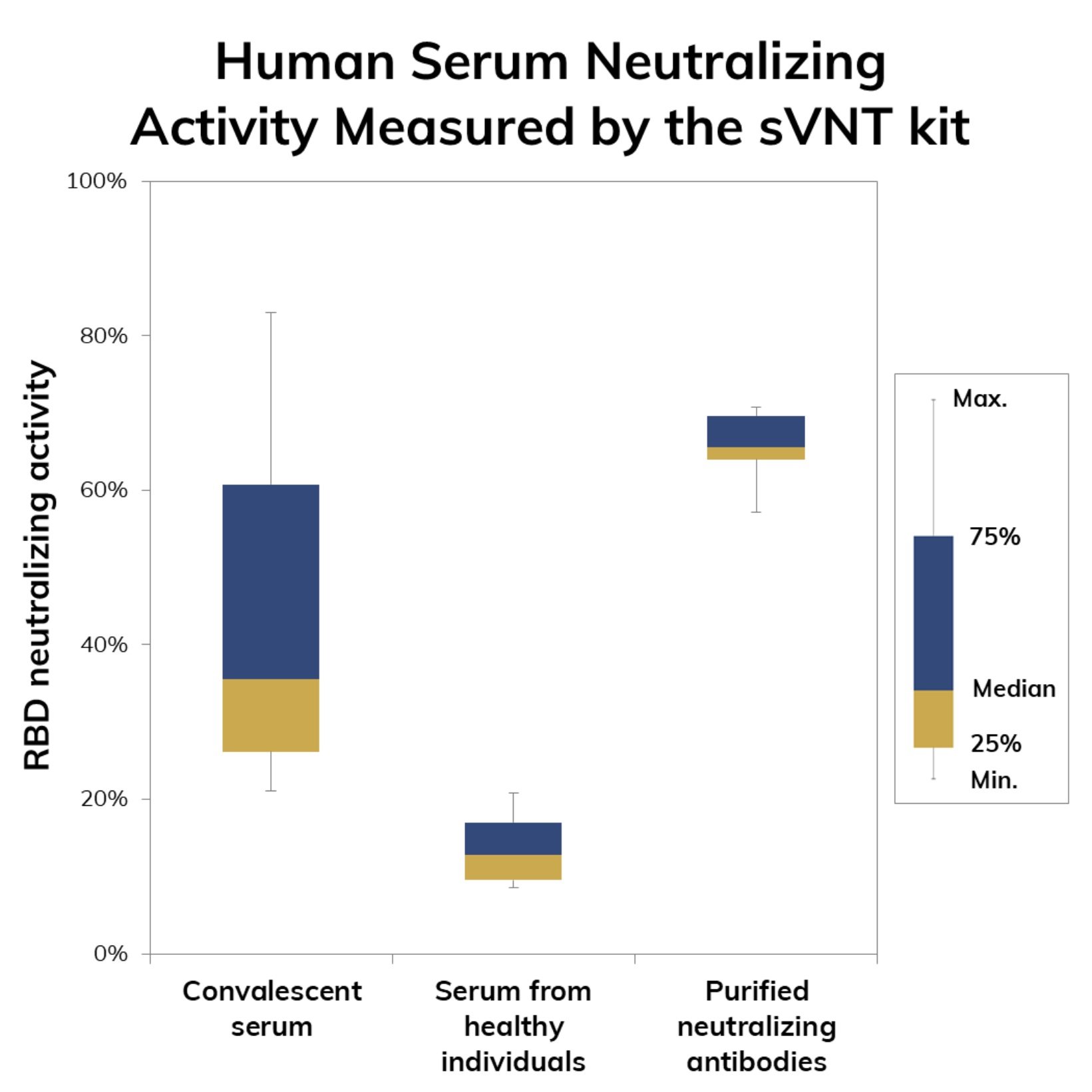
The test kit is for research use only and it can serve as a tool for immune surveillance of large populations or regions. The test also serves as a preliminary assessment of SARS-CoV-2 neutralization potential for candidate antibodies obtained either by in vivo antibody discovery technologies (e.g. hybridoma) or in vitro methodologies such as phage display.
The advantage of using the sVNT kit lies on the possibility of shortening the development times of neutralizing antibodies by reducing the number of promising molecules that need to be validated in traditional viral neutralization tests which are time-consuming such as the pseudo-virus neutralization tests (pVNT) and require access to facilities with biosafety level 3 (BSL3) such as the plaque reduction neutralization test (PRNT).
Got a question or need a quote?
Message us and we’ll get back to you 48 hours or less.
Reviews
There are no reviews yet.