 Antibody production
Antibody production
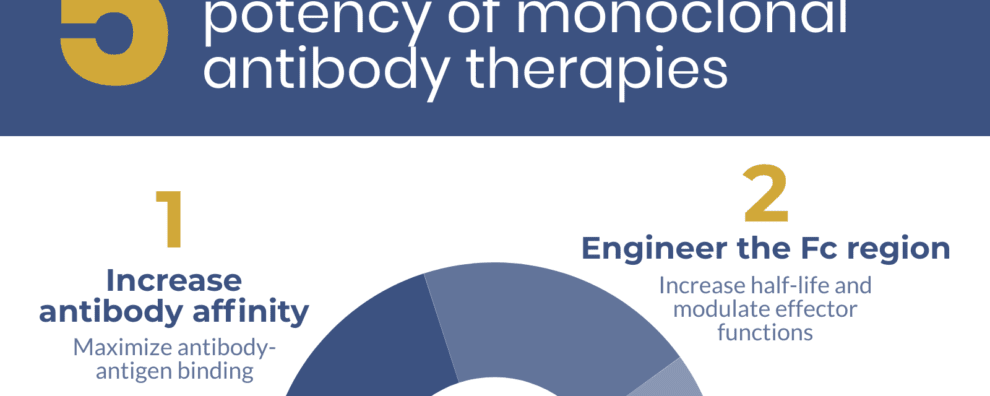
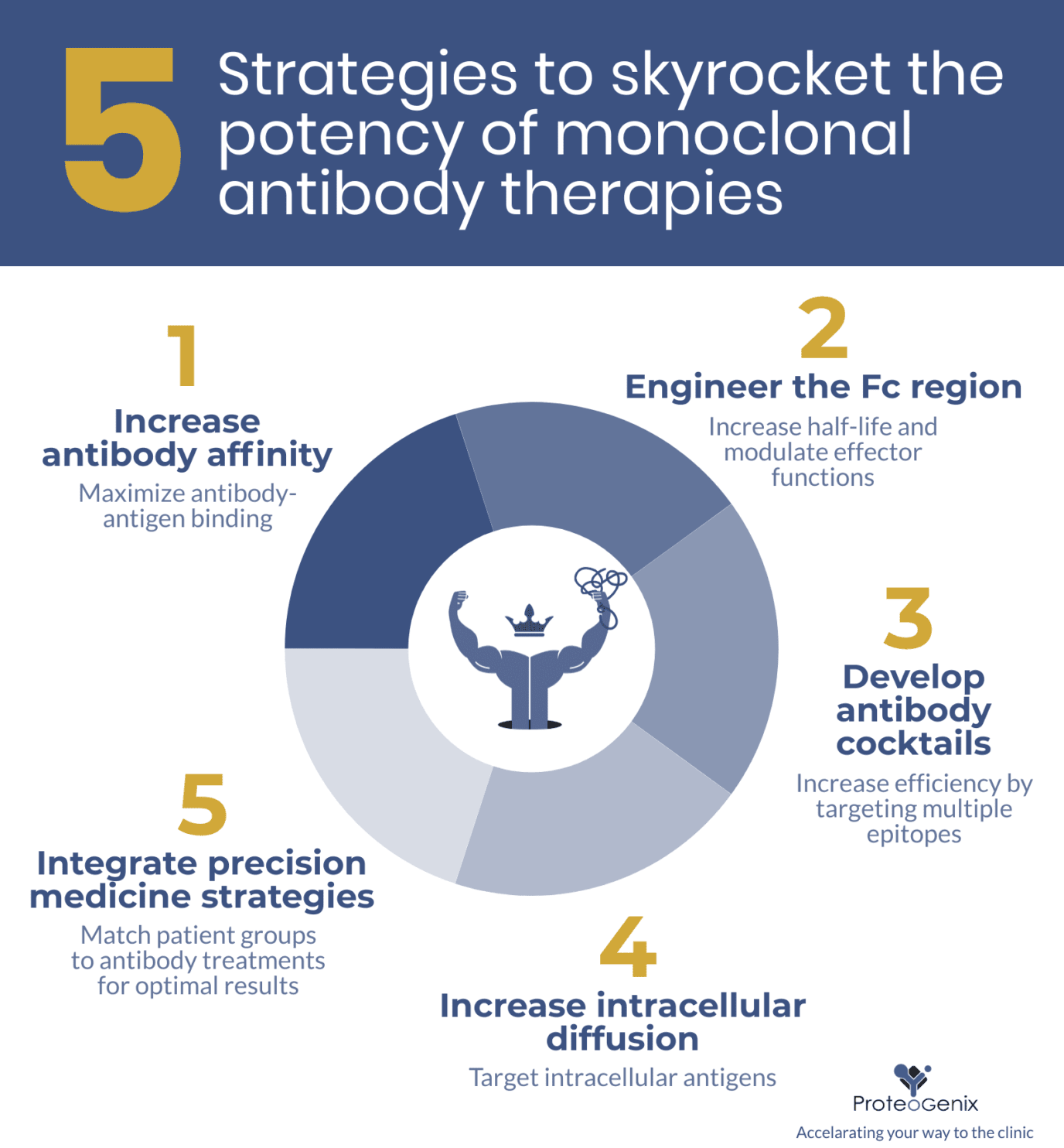
5 strategies to skyrocket the potency of monoclonal antibody therapy
Every year since 2014, at least 6 new monoclonal antibody therapies enter the clinic. These molecules are designed to target many different diseases, including cancer, autoimmune, CNS, infectious, among others. The current therapeutic limitations of mAbs continue to prompt scientists and industry leaders in the search for new strategies to boost the efficiency and reduce the cost of new immunotherapies.
1. Increase antibody affinity
Our organism’s immune response is elicited by the presence of foreign molecules, particles, or microorganisms. This primary reaction selects the binder among the naïve antibody repertoire with the best affinity towards a given antigen. The selected chains subsequently undergo a process of affinity maturation to boost their affinity.
In our organism, this process is achieved by several rounds of somatic hypermutation and clonal selection. In the lab, this process can be much more challenging and complex. Our organism’s natural ability to mature the affinity of an antibody and the technical difficulties of mimicking this process in vitro, often help to explain the general preference towards in vivo methods of antibody discovery.
However, as our knowledge of the process of antibody maturation increases, so does our ability to mimic it in the lab. Currently, in vitro affinity maturation can be achieved by random or targeted mutagenesis on the complementarity determining region (CDR) loops of an antibody. These efforts are guided by computational approaches that use protein structure and protein interaction databases to predict which iterations will be more beneficial. And acceptable candidates are screened using display technologies (i.e. phage, yeast, mammalian cell display).
However, with the advent of next-generation sequencing technologies and in silico methods, the typically time-consuming and technically-demanding approach to in vitro affinity maturation may soon change.
2. Engineer the Fc region
The effector function and half-life of monoclonal antibodies are governed by the crystallizable fragment (Fc) of these biomolecules. For this reason, Fc glycoengineering and point mutations have been recurrently used to modulate these characteristics.
Natural monoclonal antibodies have a considerably long half-life in our organism (at least 1 week) in comparison to small drugs and antibody fragments. However, the potency of a given monoclonal antibody therapy could be significantly increased if the half-life of these molecules would be further extended, providing the benefits of passive immunization for longer periods of time.
By extending the half-life of these molecules, it would be possible to reduce the frequency of injections in patients and, subsequently, reduce the currently large production requirements of these biopharmaceuticals.
Moreover, modulating the interaction of monoclonal antibodies with specific cell receptors could affect the type of immune response elicited by these molecules in our organism. For instance, certain mutations and glycoengineering of the Fc region could help enhance (or eliminate) typical effector responses such as antibody-mediated cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP), which kill targeted cells by the production of cell-death-inducing molecules or by phagocytosis, respectively.
Many studies have been published evaluating this approach, and both in vitro and in vivo results show great promise. For this reason, Fc engineering is likely to influence the development of new and more effective monoclonal antibody therapies in the near future.
3. Develop antibody cocktail therapies
Monoclonal antibody therapies have proven to be effective for cancer and autoimmune diseases. However, for other applications, such as infectious diseases, there is still a considerable room for improvement. For this reason, antibody cocktails have been often referred to as the next generation of biopharmaceuticals.
Antibody cocktails consist of a mixture of 2 or more monoclonal antibodies. They represent a compromise between conventional monoclonal antibody therapies and our organism’s natural polyclonal immune response. The use of these mixtures comprises a rational effort to overcome the natural limitations of monoclonal and polyclonal therapies. The high specificity of the former may sometimes result in a reduced efficiency when escape mutants arise during treatment. The high sensitivity of latter is often counterweighted by the low productivity, low scalability, lack of specificity, and high variability of its corresponding production techniques.
Thus, researchers have long hypothesized that antibodies cocktails may be less sensitive to mutations than conventional monoclonal antibody therapies, and less risky and more robust in comparison to conventional polyclonal antibody therapies. This hypothesis has already been successfully applied to the treatment of two well-known infectious diseases: rabies and Ebola virus.
RabiMabs (Twinrab™), a mixture of 2 anti-rabies murine monoclonal antibodies, has been approved in India in 2019 and granted Orphan Drug status by the FDA. This cocktail is at the forefront of rabies prophylaxis by providing a safer alternative to the current plasma therapy with human and equine antiserum, that risk the transmission of additional human or zoonotic infections to patients.
Another remarkable example is that of REGN-EB3. This cocktail consists of a mixture of 3 fully human monoclonal antibodies that are currently under regulatory evaluation for the treatment of Ebola virus infections. Because this cocktail was designed to target non-overlapping epitopes of the same antigen, it reduces the frequency of escape mutants. Late-stage clinical trials that took place in the epicenter of the most recent Ebola virus outbreak have proven the superior potency of the treatment in comparison to another well-known monotherapy – remdesivir (a nucleotide analog).
Although monoclonal antibody cocktails were originally tested for infectious diseases, researchers are already considering the development of cocktails that target cancer and autoimmune diseases.
4. Increase intracellular diffusion
Historically, antibody therapy has been constrained by the limited diffusion of these molecules across tissues (e.g. the blood-brain barrier) and membranes. For this reason, most therapeutic antibodies target either relevant components of the extracellular space or membrane-bound antigens. However, since at least 20% of the proteome is intracellular, more and more scientists are looking to the cytosol in an attempt to find interesting targets.
But this approach requires developing strategies to enhance the limited diffusion of antibodies across tissues. One of the most interesting approaches to increase the diffusion of antibodies is their conjugation with naturally diffusible molecules such as cell-penetrating peptides (CPPs). The fusion of antibodies with CPPs has long been hypothesized as an adequate approach. But the data concerning the feasibility of this strategy is scarce.
However, in a recent study led by Dr. Katarina Radošević and colleagues, the feasibility of this approach for antibody internalization has been carefully considered. Their work demonstrated that some CPPs could enhance the diffusion of antibodies.
Interestingly, the authors identified several key parameters to take into account when developing an antibody-CPP fusion construct. For instance, the nature and the insertion site of the CPP were important, as some types of CPPs impaired the expression of the construct and some insertion positions reduced the penetration of the construct and affected antibody affinity.
Moreover, another crucial finding of this study was that the antibody in the fusion constructs needed to target a membrane-bound antigen to penetrate the cell or tissue. For this reason, antibody-CPP fusion constructs will probably be a suitable approach if the antibody of the construct is bispecific. In this case, one arm of the antibody should target the specific cell and tissue, while the other arm should target the intracellular antigen.
5. Integrate precision medicine strategies
Precision medicine takes into account the genetic diversity among patients. Unlike personalized medicine, this approach aims at distributing individual patients among different “genetic” groups. The idea that different patients respond differently to the same treatment is not new. The first pharmacogenetic test was approved by the FDA in 2005 and, US President Barack Obama launched the Precision Medicine Initiative in 2015.
Today, precision medicine is based on artificial intelligence and bioinformatics. These tools process the genetic information of patients and organize them into different genetic groups. Moreover, this approach could be further strengthened by the inclusion of immunomonitoring techniques that leverage the high-throughput and specificity of flow cytometry and fluorescent-labeled antibodies to understand how a given treatment influences disease progression in different patients.
However, most of these types of tests aren’t usually employed during the clinical evaluation of new monoclonal antibody therapies. This suggests that patient stratification has the potential to enhance the efficiency and potency of current treatments.
Concluding remarks
Scientists and industry leaders have at their disposal an entire arsenal of strategies that can be employed to enhance the efficiency of new monoclonal antibody therapies. However, these efforts should also include robust developability assessments (i.e. bioanalytic characterization). As shown by Tushar Jain and colleagues in a seminal paper published in PNAS (2017): “the establishment of early-stage developability assessment may affect the ultimate success rate of antibodies in the clinic.” Subsequently, the identification of “red flags” in antibody characteristics have led to establishment of antibody developability guidelines and the Therapeutic Antibody Profiler (TAP) tool in a study by Matthew Raybould and colleagues (PNAS).
The identification of these key characteristics in antibody that were granted clinical approval strongly suggests that researchers should consider integrating characterization studies (e.g. bioanalytics) and advanced diagnostics and monitoring tools (e.g. flow cytometry with fluorescent-labeled antibodies) as early as possible in the antibody development pipeline. The use of these tools early in the process ensures that each antibody efficiency-enhancing strategy yields the best therapeutic results.
- Lonberg, N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol. 2008; 20(4):450-459. doi: 10.1016/j.coi.2008.06.004
- Strohl, W. E. Mouse vs Pan. The Antibody Society. July 25, 2017. Available from https://www.antibodysociety.org/mouse-vs-pan/




