 Antibody production
Antibody production
How antibody development can enhance the functionality of monoclonal antibodies for therapeutic applications
Antibody development consists in the modification and engineering of monoclonal antibodies. These techniques can be used to increase or decrease the affinity of natural monoclonal antibodies. They can also be used to decrease the immunogenicity and increase the efficiency of therapeutic antibodies. In recent years, development techniques have also been used to change the natural function of antibodies. For instance, antibodies can be engineered to bind to two different targets instead of one (bispecific antibody development). Moreover, they can be designed to deliver drugs to specific targets (antibody-drug conjugates). These modifications continue to boost the potential of antibody development for therapeutic applications.
The starting point for antibody development technologies
The term antibody development is often used interchangeably with antibody production and antibody generation; however, all of these terms refer to different stages of the antibody manufacturing pipeline. Antibody generation comprises the initially steps of antibody discovery using in vivo (e.g. hybridoma) or in vitro display technologies (e.g. phage display). Antibody development comprises different technologies that allow the improvement or modification of an antibody for different applications. And antibody production includes different techniques for the large or small-scale production of these molecules.
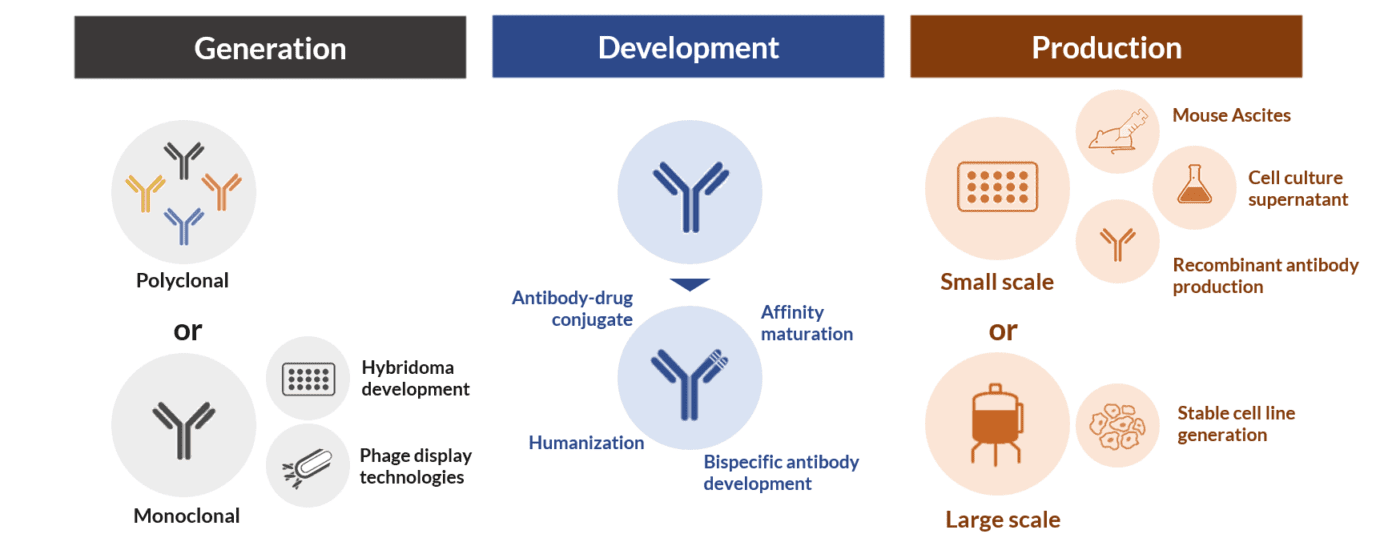
The stages of antibody production and generation are important and mandatory. But the antibody development stage is an optional step of the manufacturing pipeline. However, it is in the development stage that we can boost and change the functionality and expand the application of natural monoclonal antibodies.
The concept of antibody engineering has been proposed as early as in the 1960s. But it was with the development of molecular biology and the hybridoma technology that antibody development gained traction and credibility.
In fact, antibody development become a crucial step of the therapeutic antibody generation pipeline after the production of the first murine monoclonal antibodies by Köhler and Milstein from hybrid mouse cell lines. After this discovery, researchers soon realized these antibodies ended up challenging the patient’s immune system and causing the human anti-mouse antibody (HAMA) response which severely limited their therapeutic effectiveness.
These important drawbacks were subsequently solved by cloning of antibody genes into different expression vectors. Cloning allowed the modification of these sequences to increase their homology to the human immune system (i.e. humanization). Moreover, these recombinant technologies could subsequently be used to solve the inherent instability of hybridoma cell lines by shifting hybridoma antibody production to different and more stable expression systems.
These recombinant techniques were soon coupled with random or directed mutagenesis. Further increasing the potential to fine-tune the affinity of natural antibodies or to generate new functionalities for further applications. Within this context, it has been the development of bispecific antibodies and antibody-drug conjugates that has caused the most exciting breakthroughs of the last decade.
Bispecific antibody development
Natural monoclonal antibodies are bivalent but monospecific, i.e. they have two variable domains that can only bind a single epitope of an antigen. For this reason, they can become obsolete when the target antigen changes due to environmental conditions or antigenic drift/shift (e.g. fast-evolving virus like influenza).
The development of bispecific antibodies was proposed as early as 1960s as a way to broaden the functionality of natural antibodies. Initially, these antibodies where generated by chemical conjugation of two monoclonal antibodies.
They were originally envisaged as a way to bind tumor cell receptors and recruit cytotoxic immune cells (i.e. T cells), which would otherwise not be able to recognize the cancer cells. But it was the onset of antibody recombinant technologies that boosted the development of completely distinct and ambitious antibody structures and pushed their further application for multiple therapeutic purposes.
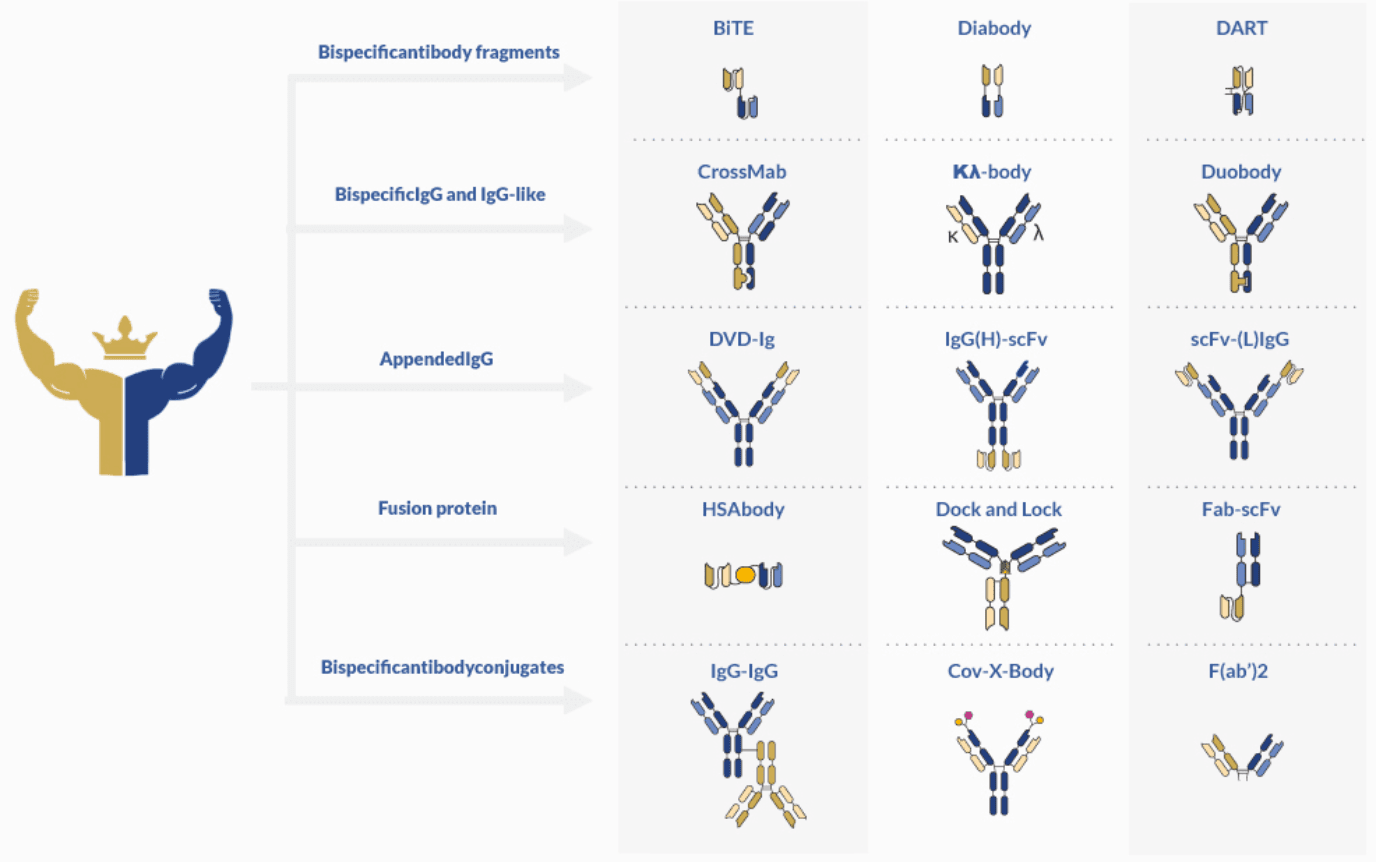
Today, the pool of synthesized bispecific antibodies comprises more than 100 different formats.
The distinctive characteristic of this type of antibodies is their ability to bind two specific antigens at the same time. This characteristic makes them invaluable tools for therapeutic and diagnostic applications. And despite the production challenges associated with bispecific antibody development, their potential for new functionalities continue to spark the interest of the scientific community.
The advantages of these antibody formats include:
- Possibility to simultaneously target multiple antigens
- Reduced risk of development of antibody-resistant variants
- Ability to function as cross-linkers (e.g. cell-surface proteins)
- Enhanced binding efficiency
- Enhanced transmembrane diffusion
These recombinant and artificial immunoglobulins can be classified according to their format and composition. The main discriminative factor is the presence or absence of constant domain (Fc).
Single-chain antibody fragments (scFvs)
scFvs are minimalist forms of a functional antibody. They are generated by the fusing of the light (VL) and heavy (VH) chains of the variable antibody domain (Fa) through the use of a glycine-rich polypeptide linker for increased flexibility.
These antibodies are about 30 kDa and lack the Fc domain. For these reasons, these small antibodies impose a minimal immunogenicity burden on patients. They can also be efficiently and quickly produced in prokaryotic expression systems (e.g. Escherichia coli), making them invaluable biotherapeutic molecules.
These antibody fragments can be further conjugated to increase stability and solubility. Currently, there are three main formats available: bispecific T-cell engager (BiTE), tandem diabodies (TandAbs) and dual-affinity re-targeting proteins (DART). BiTE molecules have found extensive use in cancer immunotherapy, for their ability to re-target T cells to tumor cells. One of the most successful BiTE drugs is blinatumomab (Blincyto®, DrugBank entry DB09052), approved for therapy by the FDA on 2014.
IgG-like bispecific antibodies
Although these antibodies are structurally more similar to natural monoclonal antibodies, they present multiple production challenges due to their engineered artificial nature. They cannot be produced or generated by natural plasma cells and, thus, one of the greatest challenges associated with bispecific IgG-like antibody production is to ensure the correct assembly of the different antibody fragments and to minimize the amount of impurities or side products (misassembled fragments).
Contrarily to scFvs, IgG-like bispecific antibodies are predominantly produced in mammalian expression systems. But due to their complex structure, the design of the expression vectors is also more complicated and time-consuming. They can be produced by different technologies including the quadroma or hybrid-hybridoma technology (fusion of two hybridomas), assembly/recombination of reduced components of two parental IgG molecules (heavy-chain assembly or heavy and light-chain assembly) and by co-culture methods (expression of two half-antibodies in two different cell lines and in vitro conjugation after purification).
The first IgG-like bispecific antibody approved for therapy was catumaxomab (Removab®, DrugBank entry DB06607) in 2009, a rat-mouse hybrid used to treat malignant ascites (cancer-associated fluids accumulation in the peritoneal cavity). And more than five new IgG-like antibodies are currently in clinical trials.
Antibody-drug conjugate development
Antibody-drug conjugates are molecules designed to deliver small cytotoxic agents to specific targets (e.g. cancerogenic cells). These molecules consist of a highly specific monoclonal antibody (natural or genetically engineered), a biologically active drug (generally cytotoxic molecules) and a stable but biodegradable linker able to release the small molecule in the target sites.
Several conjugation techniques are currently available. However, chemical conjugation is the most commonly used strategy for the development of these complex molecules. It can be performed using natural monoclonal antibodies or in engineered immunoglobulins with increased affinity to the cross-linker. However, the use of natural antibodies remains highly desirable due to the low generation costs associated with these molecules in comparison with their engineered counterparts.
These antibodies are easier to synthesize in comparison to bispecific antibodies. However, most important drawbacks associated with antibody-drug development fall upon off-target cytotoxicity and, for this reason, only a small number of these conjugates has reached the market.
Concluding remarks
Bispecific and antibody-drug conjugates are the two most prominent antibody development techniques that are currently employed for expanding the functionality of natural monoclonal antibodies. Both these techniques present different technical challenges that continue to hinder their commercialization and therapeutic application.
However, their enhanced versatility continues to spark the interest of the scientific community and to prompt the development of different recombinant molecules for increasingly ambitious therapeutic applications.
- Bratt, J. et al. Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 1. BioProcess International. 2017. Available from: https://bioprocessintl.com/manufacturing/emerging-therapeutics-manufacturing/therapeutic-igg-like-bispecific-antibodies-modular-versatility-and-manufacturing-challenges-part-1/
- Brinkmann, U. and Kontermann, R. E. The making of bispecific antibodies. mAbs. 2017; 9(2):182-212. doi: 10.1080/19420862.2016.1268307
- Chames, P. et al. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009 May; 157(2): 220–233. doi: 10.1111/j.1476-5381.2009.00190.x
- Köhler, G. and Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256:495–497. doi:10.1038/321522a0
- Nejadmoghaddam, M. R. et al. Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna J Med Biotechnol. 2019; 11(1): 3–23. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6359697/
- Wang, Q. et al. Design and Production of Bispecific Antibodies. Antibodies. 2019; 8(3)-43. doi: 10.3390/antib8030043




